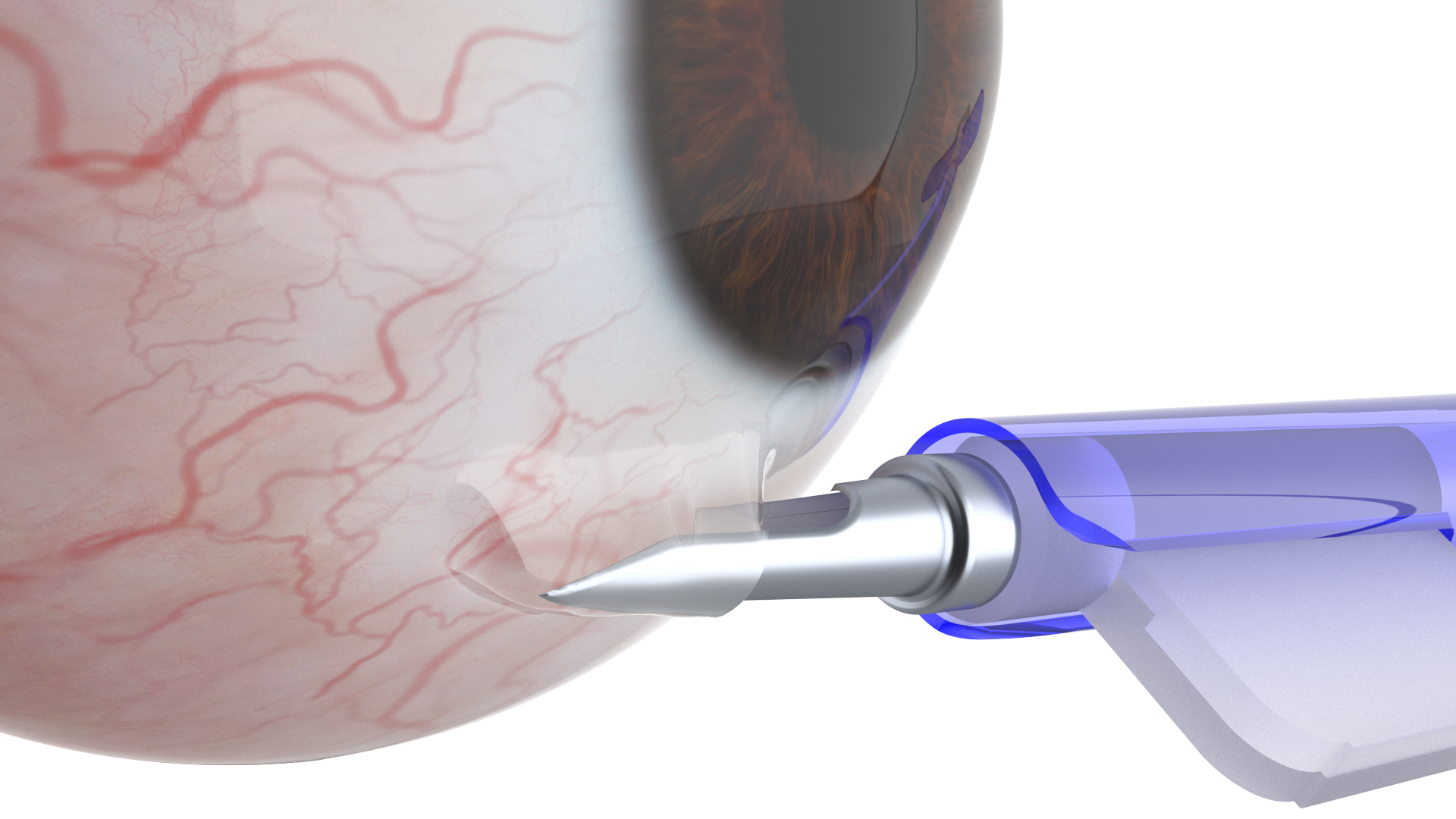
Vi-Sci’s innovative Eye-D technology treats glaucoma by the insertion of a sub-conjunctival implant, which releases the prescribed medication “Latanaprost”, in sustained release manner, eliminating the need to use eye drops, a daily challenge for the aged.
The implant; an in-office medical procedure is developed by ViSci, branded as VS-101, is licensed for global use and is in its development phase.
ViSci’s Market
The global glaucoma drug market is expected to cross the $10 billion mark by 2026, with the most significant growth expected in developing countries and areas such as China, India and South America. Glaucoma prevalence increases with the natural increase in population and even more so, due to the prolongation of the average life expectancy and the resultant increase in background diseases, including glaucoma.
Two known and conflicting glaucoma-related problems are the basis of the business model for the product: on the one hand, glaucoma is the most prominent global cause of irreversible blindness and on the other hand, patients’ levels of persistence with recommended and effective drug treatment are unusually low and reach, according to a number of studies conducted in the field, no more than about 25% about one year after diagnosis. This paradox is pushing many companies to seek a solution that will reduce patients’ treatment burden without impacting the efficacy of the treatment and without raising the level of risk associated with treatment, yet maintaining economic logic for insurers and healthcare systems.
The solutions developed by ViSci reduce the burden of treatment for glaucoma patients.
About the Solution
The implant administers medication in controlled release, sufficient for a period of up to one year of treatment, during which patients are spared the burden of daily use of eyedrops without, of course, impacting the treatment’s efficacy.
The doctor places the implant under the conjunctiva in the clinic, in a simple and short procedure, by a dedicated implanter developed by the company. The patient does not feel the implant and it cannot be seen by others.
The company has long since successfully completed, in the United States, a semi-blinded random trial: the blinded part included three treatment arms of implants with different doses and a non-blinded reference arm of eyedrops (Xalatan® – the most common generic product for treating glaucoma).
- The trial results clearly demonstrated the safety of the implant use.
In addition, the effect of the pace of release of the active ingredient was clearly presented (see graph).
ViSci is actively seeking a financing partner for the next clinical trial required in the development process.