DiagnosTear
leading ophthalmic company developing and commercializing disruptive diagnostic solutions

DiagnosTear is a leading ophthalmic company developing and commercializing disruptive diagnostic solutions for better management of eye diseases.
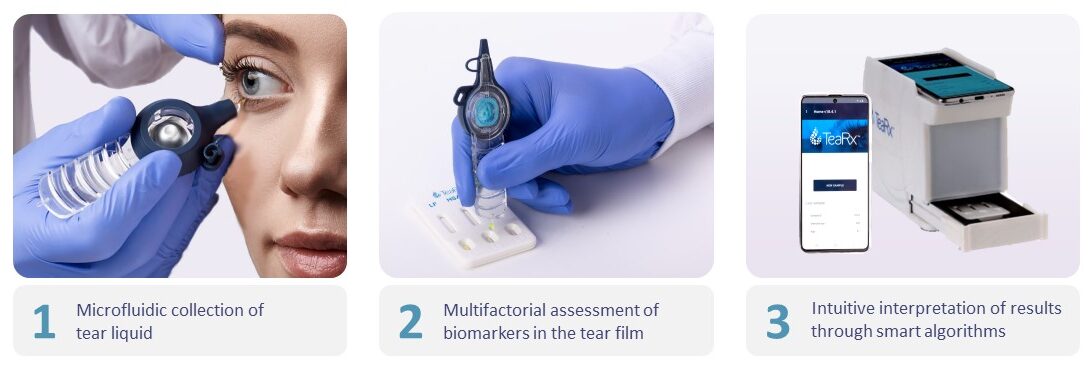
DiagnosTear’s TeaRx™ technology is a diagnostic platform intended for Point-of-Care Testing (POCT) of ophthalmic pathologies through multi-parameter composition analysis of the tear fluid.
TeaRx™ technology is based on a minute sample of tear fluid taken with a unique, single-use, microfluidic tear collection device, followed by a semi-quantitative multi-parameter immunoassay, visual or digital readout and cloud-based analysis providing intuitive interpretation of the results. The whole procedure takes less than 10 minutes, is easy to perform and apart from the hand-held test reader (supplied by DiagnosTear), does not require sophisticated, expensive, or space-consuming instrumentation.
The first CE-IVD approved test based on the TeaRx™ platform is intended for the identification and monitoring of Dry Eye Syndrome (TeaRx™ DES).
DES is a common disorder in which there is a decline in tear production or tear quality is impaired. Moderate and severe DES may cause pain and discomfort, and impair vision quality. In extreme cases, DES may even cause permanent blindness. Some 340 million people worldwide have DES, including some 40 million in the United States. Symptoms associated with DES account for about one third of patients’ complaints to ophthalmologists in the United States.
Despite its widespread prevalence, DES is complex and difficult to diagnose and treat since it may be caused by many underlying factors, resulting in diverse and distributed sub-populations suffering from the syndrome. Tear fluid is composed of a great many substances and a change in their concentration indicates a problem with the quality or volume of tears that causes the syndrome.
Multi-parameter analysis of the tear film proteins provides additional insights as per the etiology of the disease (e.g., evaporative, aqueous deficient, inflammatory DES) and may assist the ophthalmologist in prescribing the optimal treatment matched to the underlying cause of the disease.
The clinical performance of the test was assessed in four clinical trials performed in the US and in Israel. Sensitivity and specificity levels exceeded 86% for detection of high severity DES. Interim results from a large (600 subjects) clinical trial conducted in India, indicate approximately 80% sensitivity for identification of DES at all severity levels as a single predictive device.
Beyond DES, DiagnosTear is developing innovative tests based on the TeaRx™ platform for additional ophthalmic indications. Among others, DiagnosTear’s pipeline tests include TeaRx™ Red Eye; The first test of its kind for differential assessment of adenoviral conjunctivitis, Herpetic Keratitis and Allergic conjunctivitis. This test is intended to be used by primary care physicians (GPs, family practitioners and pediatricians) as well as by ophthalmologists and is envisioned to allow on-site diagnosis of viral vs. allergic Red Eye and aid the physician in prescribing the optimal treatment and/or refer the patient for further examinations (e.g., fluorescein/Rose-Bengal corneal staining and/or laboratory testing by PCR/culture).
For more information about us, our vision and the TeaRx™ technology, please click “Company Presentation” here.